Methodological Considerations Regarding the Quantification of DNA Impurities in the COVID-19 mRNA Vaccine Comirnaty®
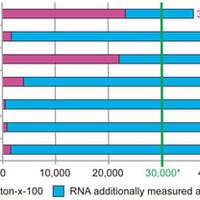
DNA impurities can impact the safety of genetically engineered pharmaceuticals; thus, a specific limit value must be set for them during marketing authorisation. This particularly applies to mRNA vaccines, as large quantities of DNA templates are used for their production. Furthermore, when quantif…